Abstract
Introduction
According to population-based Surveillance, Epidemiology and End Results (SEER) cancer registry data, approximately 40% of adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL) in the United States (U.S.) are Hispanic and 7% are Black and both Hispanic and Black patients have significantly inferior outcomes relative to non-Hispanic Whites (NHW). CALGB 10403, a prospective Phase II clinical trial conducted by the U.S. Intergroup, demonstrated the feasibility of delivering a pediatric-inspired ALL regimen to newly diagnosed AYA ALL patients and found this approach to be superior to historical adult regimens (Stock et al. Blood 2019). In the current analysis, we evaluate enrollment patterns of Hispanic and Black AYAs on CALGB 10403 and compare survival relative to SEER estimates.
Methods
Demographics on AYAs (18-40 yrs) enrolled on CALGB 10403 (N=295 from 2007-2012) were supplemented with patient-reported education and socioeconomic status (SES) information. Data from the North American Association of Cancer Registries (NAACCR; 2013-2017) were used to evaluate U.S. country-wide ALL incidence (20-49 yrs) overall and among Hispanics, reported per U.S. state. SEER registries (2008-2012) provided racial/ethnic distribution and overall survival (OS) of AYA ALL (15-39 yrs). The distribution of OS was estimated using the Kaplan-Meier method and compared across racial/ethnic groups using log-rank tests.
Results
Relative to the U.S. AYA ALL population, CALGB 10403 included proportionally fewer Hispanics (41.7% vs. 16.3%, P < 0.001); the distribution of blacks was similar (6.4% vs. 8.5%, P = 0.174). Demographics and ALL characteristics among CALGB 10403 enrollees demonstrated no significant differences in age, sex, cytogenetics, or BMI by race/ethnicity; however, Black AYAs were more likely to have T-ALL, and Hispanic patients were more likely to have Ph-like B-ALL and rearrangements in CRLF2. Hispanic and Black patients reported lower household yearly income, and Black patients had less educational attainment.
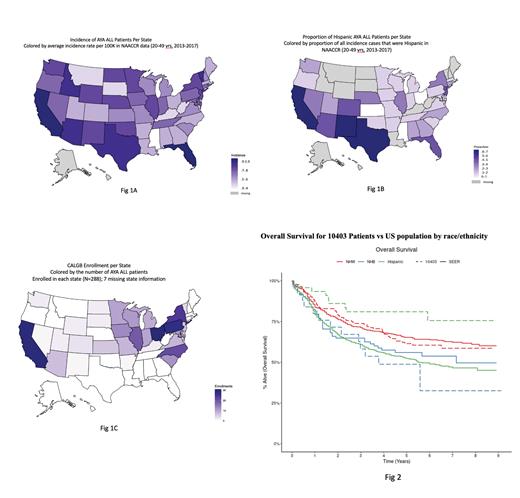
In order to explore reasons for disproportionately low Hispanic enrollment on CALGB 10403, we calculated the proportion of Hispanic AYAs with ALL relative to the AYA ALL incidence across U.S. states using NAACCR data and compared this with the geographical distribution of CALGB 10403 enrollments. The incidence of AYA ALL across U.S. states is represented in Fig 1A and the proportion of Hispanic AYA ALL patients in Fig 1B. The states with highest proportion of Hispanic patients included CA, TX, and NM, followed by AZ, CO, FL, and NJ. Although CALGB 10403 enrolled patients in 31 U.S. states, the trial did not open in TX or FL. The enrollments numbers were highest across the Mid-Western U.S., with only 47/295 (15.9%) of enrollments coming from the seven states with a high proportion of Hispanic AYA ALL patients (Fig 1C).
The 3-yr event-free survival (EFS) and OS for the total CALGB 10403 cohort were 59.1% (53.1%, 65.75%) and 73.5% (68.1%, 79.4%), respectively. Per protocol completion of therapy was highest in Hispanics (75%), relative to Black (64%) and NHW patients (56%), P=0.049. Overall survival among CALGB 10403 participants differed significantly across race/ethnicity (Fig 2), with superior OS experienced by Hispanic patients and inferior OS by Black patients (P= 0.021). These clinical trial results differ from SEER estimates, where both Hispanic and Black patients experience worse OS relative to NHW (Fig 2).
Conclusions
This in-depth analysis of racial/ethnic enrollments and outcomes on CALGB 10403 uncovered findings that may translate across clinical trials beyond AYA ALL. Importantly, our data suggest that closer matching of trial site selection to geographical racial/ethnic disease incidence may improve minority enrollments on multi-center clinical trials. Relative to NHW, Hispanic patients had superior survival despite having higher rates of Ph-like ALL, indicating that clinical trial participation may mitigate racial/ethnic survival disparities seen in population-based data. Finally, we saw no improvement in trial outcomes among Black AYAs; a deeper understanding of the biological leukemia underpinnings and barriers to successful outcomes would aid future efforts at improving survival across racial/ethnic groups.
Muffly: Pfizer, Amgen, Jazz, Medexus, Pfizer: Consultancy; Astellas, Jasper, Adaptive, Baxalta: Research Funding; Adaptive: Honoraria, Other: fees for non-CME/CE services: , Research Funding. Advani: Abbvie: Research Funding; Macrogenics: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Glycomimetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunogen: Research Funding; Pfizer: Honoraria, Research Funding; OBI: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Luger: Syros: Honoraria; Agios: Honoraria; Daiichi Sankyo: Honoraria; Jazz Pharmaceuticals: Honoraria; Brystol Myers Squibb: Honoraria; Acceleron: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Onconova: Research Funding; Celgene: Research Funding; Biosight: Research Funding; Hoffman LaRoche: Research Funding; Kura: Research Funding. Tallman: NYU Grand Rounds: Honoraria; Syros: Membership on an entity's Board of Directors or advisory committees; Kura: Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Biosight: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Oncolyze: Membership on an entity's Board of Directors or advisory committees; KAHR: Membership on an entity's Board of Directors or advisory committees; Orsenix: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Rafael Pharmaceuticals: Research Funding; Glycomimetics: Research Funding; Biosight: Research Funding; Orsenix: Research Funding; Abbvie: Research Funding; Mayo Clinic: Honoraria; UC DAVIS: Honoraria; Northwell Grand Rounds: Honoraria; NYU Grand Rounds: Honoraria; Danbury Hospital Tumor Board: Honoraria; Acute Leukemia Forum: Honoraria; Miami Leukemia Symposium: Honoraria; New Orleans Cancer Symposium: Honoraria; ASH: Honoraria; NCCN: Honoraria. Litzow: Omeros: Other: Advisory Board; Jazz: Other: Advisory Board; Pluristem: Research Funding; Actinium: Research Funding; Amgen: Research Funding; AbbVie: Research Funding; Astellas: Research Funding; Biosight: Other: Data monitoring committee. Foster: Macrogenics: Research Funding; Rafael Pharmaceuticals: Research Funding; Macrogenics: Consultancy; Daiichi Sankyo: Consultancy; Agios: Consultancy; Bellicum Pharmaceuticals: Research Funding. Larson: Gilead: Research Funding; Epizyme: Consultancy; Astellas: Consultancy, Research Funding; Rafael Pharmaceuticals: Research Funding; CVS/Caremark: Consultancy; Takeda: Research Funding; Novartis: Research Funding; Cellectis: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal